Oncology and Hematology Expertise
Patient-centric oncology CRO services
Oncology and hematology studies dominate the clinical trial landscape, but the path to regulatory approval is complex and uncertain. And for the millions of patients each year facing a cancer diagnosis, the search for answers can’t wait.
While advancements in oncology and hematology clinical research bring hope to patients, they can present challenges to drug development, including:
- Complexity of innovative trial designs and master protocols
- Enrollment barriers that hinder diversity and inclusion goals
- Intense competition for qualified clinical trial sites and patients
- Higher data volume and collection requirements compared to other therapeutic areas
- Rapidly changing medical standards of care and clinical strategies
- Regulatory and payor requirements that necessitate long-term follow-up
To navigate these complexities, you need a nuanced clinical development strategy and the partnership of an experienced global provider of contract research organization (CRO) solutions with patient-centric technologies and end-to-end solutions and services that span all phases of development.
With experience in more than 700 oncology and hematology global clinical trials, the PPD™ clinical research business of Thermo Fisher Scientific has expertise across the full range of cancer therapies — from immuno-oncology and targeted therapies to novel and emerging therapies, including cell and gene therapies.
Accelerating oncology and hematology clinical research with proven experience
For more than 30 years, our CRO solutions have enabled drug developers to develop and commercialize their oncology and hematology treatments. Success in oncology and hematology clinical research requires a provider of CRO solutions with a deep understanding of the disease and an unrelenting dedication to developing the services that patients need. In the past five years alone, our team has supported 34 oncology drug approvals in the U.S. — including three first-in-class products — and 25 EMEA approvals.
studies
patients
sites
countries
Our areas of expertise include the following:
Novel modalities
Novel oncology drug development has seen an increasing focus on targeted drugs with innovative mechanisms of action for treatment of cancer over the last decade.
The various categories of novel and therapeutic modalities in oncology include antibodies, proteins, peptides, cell therapies, gene therapies, nucleic acids and radiopharmaceuticals.
Our oncology CRO services are here to help you navigate these evolving medical therapies and help move your drug development programs forward.

Radiopharmaceuticals
Radioligand therapy (RLT) is a revolutionary new approach to cancer treatment. It is becoming a crucial component in oncology and cancer care by offering a personalized approach to treatment, which incorporates both diagnostic and therapeutic tools to enhance treatment outcomes and patient care.
We have extensive experience partnering with clients to advance the development of radioligand therapy in oncology trials across several disease areas and development phases.
Learn more about best practices for the successful execution of radiopharmaceutical clinical trials

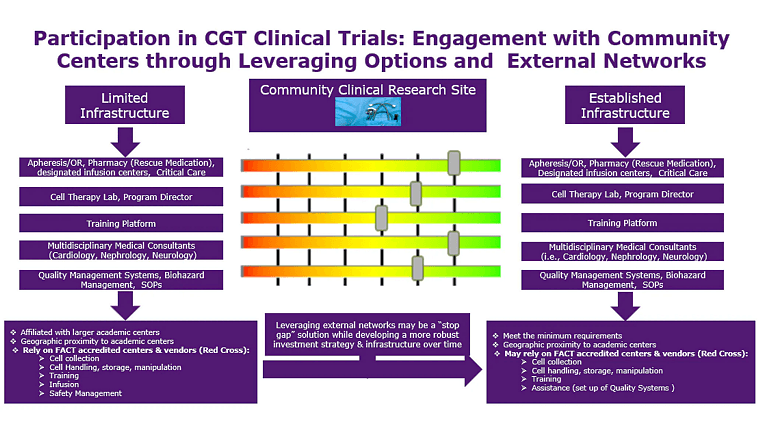
Cell and gene therapy
When it comes to cell and gene therapy (CGT) clinical trials, you need to put your trust in expertise and an experienced provider of CRO solutions. We have spent decades on the development and management of CGT trials, including 160 immuno-oncology studies and 137 cell and gene therapy trials across tumor types and therapies.
Over the years, our oncology CRO services have supported more than 33,000 patients, and we offer comprehensive laboratory support for cell and gene therapies, including CAR-T therapies.
Adoptive cell therapies comprise one of the largest classes of novel immunotherapies in oncology. While interest among drug developers continues to grow, the adoptive cell therapy development ecosystem is complex and requires continual learning and process refinement. As technology advances and impactful oncology therapies are developed, drug developers will need an experienced provider of CRO solutions to support oncology clinical trials and manage logistics.
Explore the key challenges of cell therapy clinical trials and strategies for increasing patient access.
Discover emerging trends and regulatory hurdles.

Immuno-Oncology Center of Excellence
Immuno-oncology therapies present an area of opportunity in oncology and hematology drug development. New immunotherapies, which are demonstrating improved median overall survival and an acceptable toxicity profile, offer evidence for optimism.
With our Immuno-Oncology Center of Excellence, we provide innovative oncology solutions and enable drug developers to pursue their oncology therapies with confidence. We provide a knowledge base on the competitive environment, efficient adaptive designs, and the dynamic oncology regulatory landscape that surrounds these rapidly evolving immunotherapies.
Our oncology experience spans more than a decade and includes more than 160 clinical trials on adoptive cell therapies, cancer vaccines, checkpoint inhibitors, cytokines, immune modulators and monoclonal antibodies.
More than 33,000 patients have participated in these oncology trials at more than 5,000 sites worldwide, and our experience with cell and gene therapy clinical trials continues to grow, with a background in more than 30 CGT studies. Our Cell and Gene Therapy Institute offers cross-functional expertise that spans diverse technologies and delivery mechanisms, including cell, gene, gene-modified cell and advanced tissue technologies.
It also offers cross-functional expertise with oncology and hematology physicians well-versed in CAR-T therapies with investigators and key opinion leaders at the top of their field.

Rare diseases in oncology and hematology
Rare disease in oncology and hematology encompass a wide range of conditions that affect the blood, bone marrow, and various organs, often involving complex and life-threatening symptoms. Given the wide range of indications, including rare cancers, uncommon blood malignancies, and benign hematologic conditions, it is of paramount importance to understand the nuances of each to develop effective strategies.
Our team of specialists provide comprehensive support for clinical trials that include low incidence cancers as well as benign and malignant hematological conditions that cover all stages, from first in human to post-registration clinical trials.
We are experienced in the management of a spectrum of hematological indications, including lymphoma, acute myelogenous leukemia (AML), myelodysplastic syndrome (MDS), multiple myeloma, and benign and malignant disorders.

Advancing pediatric cancer research
Pediatric oncology and hematology expertise is essential to developing and commercializing therapies for children. Oncology and hematology clinical trials are complex, and these challenges increase with a pediatric population. It is important to use an experienced provider of oncology CRO solutions in the management of pediatric trials.
Our experts guide you through the careful planning and consideration needed for pediatric oncology clinical trials. With experience across all phases, oncology indications and therapeutic areas — including more than 500 clinical studies — the cross-functional team at our Rare Disease and Pediatrics Center of Excellence delivers innovative, patient-centric solutions to skillfully address the most complex challenges of these oncology trials.

Innovative trial designs
The application of innovative oncology trial designs — such as adaptive designs and master protocols — can have a big impact in early phase oncology trials when decisions have far-reaching consequences.
Adaptive designs can transform your study by leveraging accumulating data to answer multiple questions and inform decisions in a flexible process. The ability to modify your early-phase oncology clinical trials as they progress allows for accelerated timelines, reduced costs, and improved quality and efficiency in decision-making.
With adaptive clinical trial design, potential issues are revealed earlier, enabling faster “go, no-go” decision-making and more confidence in outcomes.
We are experts in innovative adaptive clinical trial designs and master protocols. Our proven oncology experience designing and operationalizing adaptive and other innovative clinical trial designs, through our Adaptive Design and Master Protocol Working Group, enables you to unlock better information, faster.

Early phase oncology
We are a provider of early phase CRO solutions that delivers operational expertise in the clinical development of early phase oncology assets. Our expertise is founded on proven strategies, with on-demand access to critical and supportive resources tailored to early phase oncology trials.
Our dedicated team includes global experts in early clinical development oncology and clinical pharmacology. They perform a critical assessment of your compound’s unique attributes in order to identify areas of strength and potential weakness that could impact the outcome of your current and subsequent oncology clinical trials. The team develops an integrated, early-phase oncology program that leverages our facilities, site network, operational expertise and development experience to execute your oncology trial in patients and/or healthy volunteers. Each step of the process is conducted with an eye toward supporting later-phase programs and regulatory submissions.
- Access to a global network of early-phase research sites that conduct studies in general and specialty populations
- A range of consulting services for asset planning in the earliest phases of development and regulatory strategy assessment to mitigate risk
- A dedicated team of early development professionals in our early development services group, helping to execute Phase I and IB trials that accelerate your trial and contain costs
- Support for early phase oncology trials, including customized assays for drug product evaluation, as well as bioanalytical and pharmacokinetic assays

Biosimilars
Partner with us to develop biosimilar products more quickly and cost-effectively, from clinical and operational planning to pipeline prioritization, feasibility analyses and full-service clinical study execution.
As experts in biosimilar development, we understand your challenges and sense of urgency. We have the proven clinical expertise to help you design a sound development plan and regulatory strategy to ensure the successful execution of your biosimilar program.
Our goal is to help overcome clinical challenges and expedite full development to bring your biosimilar to market faster. We offer a full range of biosimilar clinical drug development services, from cell line development and characterization to clinical development and market approval.

Biosimilar product development services:
- Preclinical development
- Startup
- Global clinical supplies
- Biosimilar investigator network
- Clinical development
- Regulatory affairs
- PK/PD
- CMC support
- Biostatistics
Some of our achievements include:
- Supported the development of all top-10 selling biologic products
- Successfully delivered the first monoclonal antibody biosimilar to the EU market
- PPD Laboratory services Bioanalytical lab has developed assays and tested more than 300,000 samples
- Proven track record of accelerating enrollment in biosimilar studies
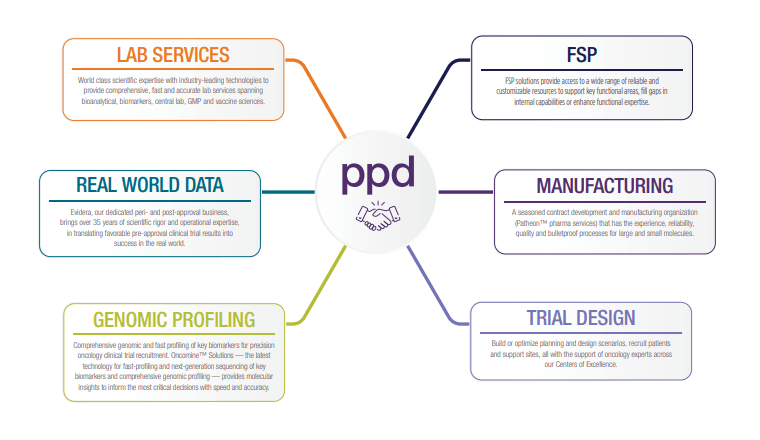
Explore our end-to-end oncology capabilities
Comprehensive oncology and hematology laboratory services
Across every phase, PPD™ Laboratory services deliver the quality data needed for fast, accurate decision-making. Our oncology and hematology portfolio spans:
- Antibody-drug conjugates (ADCs)
- Biologics
- Biomarkers
- Cell and gene therapies
- Companion diagnostics
- Small molecules
Our collaboration with NeoGenomics expands oncology testing for clinical trials in areas such as genetic testing, pathology testing and interpretation services.