Gastroenterology
In-depth clinical trial expertise in GI and hepatology disorders
The PPD™ clinical research business of Thermo Fisher Scientific partners closely with customers across a number of gastroenterology disorders. Our team is rich in clinical talent with physicians who understand the progress needed to treat indications in this disease. The gastroentrology team has performed trials for the majority of top 10 pharmaceutical companies and for emerging biotech companies.
We have an established track record of delivering, with many of the studies we have managed enrolling and completing early in a competitive environment. With a dedicated staff of experts with broad experience, our gastroenterology clinical trials are executed successfully and innovatively with each customer’s goal in mind.
A confluence of factors has spawned an exponential increase in gastrointestinal and hepatic disorders that are taking their toll on quality of life and life expectancy for millions of people worldwide.
Your compound may be the first to have a measurable impact on hundreds of millions of people with metabolic dysfunction-associated liver disease (NASH), or a greater efficacy and safety for millions more who suffer from inflammatory bowel disease and functional gastrointestinal (GI) disorders.
Our experience in the past five years:
Gastroenterological trials
Global sites
Global patients
Access to patients that launches your study faster
We own a large patient database of fully identified and opted-in patients, plus a global network of 180+ dedicated research sites worldwide.
Because we know the patients and we own the sites, we can accurately forecast and offer patient enrollment and budget certainty. This virtually eliminates the usual uncertainty surrounding patient numbers, site performance, enrollment timelines and costs. Moreover, it allows us to launch your study faster, with quality patients, using fewer sites that perform at maximum capacity.
Connecting with IBD patients with COLO Flare
IBD (inflammatory bowel disease) studies are extremely competitive, and we offer our customers a competitive edge. Through IBDConnect, we bring your IBD study directly to patients at their own GI physician’s office instead of solely relying on active research sites to source patients.
And with our COLO Flare app, pre-screened patients can quickly report IBD flares and other study-specific criteria via an app, to their local site, so they can be immediately screened for your study. Patients remain engaged until flare symptoms emerge, and then they’re directed to their site coordinator to discuss study participation.
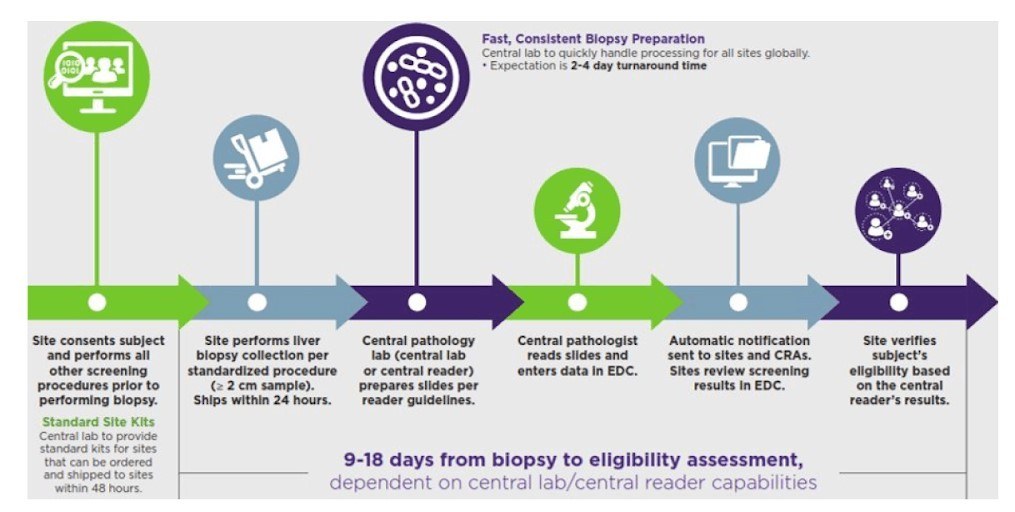
Clear, accurate biopsy results for NASH
Because clear, accurate biopsy results are critical for your study, we provide each site with standardized biopsy collection and slide preparation procedures. We coordinate patients’ schedules, submission of samples to the central reviewer or pathologist, and results reporting.
NASH expertise
To better understand the NASH patient population, our AES team surveyed 441 adults with fatty liver disease, hypertension, high cholesterol, type 2 diabetes and obesity about their conditions and their perceptions toward clinical trial participation. The results formed the basis of our recruitment strategies, enabling us to target and identify the most qualified patients closest to study sites.
- Deep understanding of medical and regulatory requirements
- Ability to provide consultation on all aspects of the study
- Strong site relationships and experience with fast-enrolling sites
- Proven rapid startup with known NASH sites
- Refined patient pathway for efficient diagnosis process, ensuring maximum patient reach
- Experience with novel imaging techniques and managing liver biopsies