Archives: Resources
Implementation Science Webinar
Watch this webinar to learn how the emerging field of implementation science helps overcome new healthcare innovation challenges.
Contract Pharma 2023 CRO Industry Report
Explore key insights, challenges and trends in the global CRO services market.
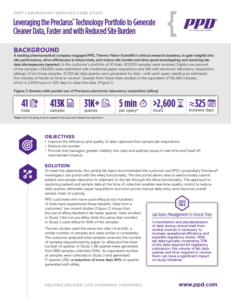
Building a Strong Data Foundation for DCTs with an FSP Model
Access four key decision points critical for optimizing your decentralized trial.
Conversion to a Sole-provider FSP Model
Learn how our FSP experts delivered process improvements that outperformed customer goals.
Case Study: How to Reduce Site Burden and Lab Data Queries Using Preclarus™
See how Preclarus enables more-informed decisions and significant resource and time savings.
Defining Demographic Cohorts in Clinical Trial Populations
Learn how to achieve appropriate diversity in clinical trials by using real world data in this Contemporary Clinical Trial paper.
Solving Challenges in Cell Therapy Clinical Trials & Effectively Delivering Complex Studies in Advanced Therapeutics
BioInsights interviews PPD immuno-oncology experts for their insights on overcoming key obstacles for developing cell therapies in oncology.
Patient Centricity in Clinical Trials
Dig into impactful methods that drive patient centricity and diversity in this on-demand webinar.
Case Study: How to Reduce Site Burden and Lab Data Queries Using Preclarus™
Pharmaceutical and biotechnology companies often need to gain insights into site performance, drive efficiencies in future trials, and reduce site burden and time spent investigating and resolving lab data discrepancies (queries).
Key to improving data quality and clinical trial efficiency is Preclarus™, PPD’s comprehensive clinical data portfolio solution that consolidates and presents data from multiple sources. Using the Preclarus eReq functionality, a company can see up to an 80 percent reduction in the number of queries – a potential savings of hundreds of business days.