
PPD Laboratory Services, GMP Lab
GMP pharmaceutical testing solutions and services
Our good manufacturing practice (GMP) lab provides high-quality analytical services for small molecules and biologics including active pharmaceutical ingredient (API), drug products, inhaled products and other medical devices for all phases of drug development. Our GMP lab team is a leading provider of chemistry, manufacturing and controls (CMC) lab services, with three decades of experience, and focuses on responding to clients’ needs with the latest technology, quality service and a high degree of flexibility. Our services meet the requirements of regulatory authorities worldwide.
We perform CMC testing following current good manufacturing practices (cGMP) for all types of pharmaceutical products across the phases of development. Our GMP lab scientists work with small molecules and biologics, including APIs, drug products, inhaled products, cell and gene therapies and drug-device combination products.
State-of-the-art facilities and extensive experience
Shorten your development timelines with our GMP lab

Laboratory services with three decades of GMP & CMC testing experience – from early development characterization, formulation support analysis, stability testing and method development and method validation, to GMP batch release testing.
Read the white paperState-of-the-art facilities and experienced personnel supporting:
- Analytical development and validation for drug products and devices, starting materials, APIs, etc.
- Stability testing and storage
- Release and quality control testing
- Inhaled pharmaceutical and biologics testing
- Physiochemical characterization
- Specialized expertise in mass spectroscopy
- Potency bioassay and molecular biology applications
- Microbiology
- CMC advisory services
- Impurity identification and characterization
- Qualified person (QP) release services
- Infectivity
Our GMP lab met or exceeded expectations for quality of analytical testing for 98% of our customers
We are ready to partner with you
Our GMP lab, by the numbers
Founded three decades ago, our GMP lab has grown to more than 15 times its original size and has significantly expanded its service offerings. With locations in the U.S. (Wisconsin) and Europe (Ireland), our GMP lab has a deep-rooted industry commitment and strong global presence to keep pace with growing and changing industry needs.
square feet of lab space in the U.S and Europe
scientists and staff across the globe
years of meeting customers’ CMC testing needs
We’re growing. Read our recent news release

The industry standard of GMP labs
Our specialized, cross-functional teams draw upon their deep industry and CMC pharmaceutical testing experience to create an innovative plan for your program that satisfies or exceeds regulatory, timeline and budget requirements. We collaborate with your team from the start to fully understand your goals, explore the most appropriate technology and methodology, and provide critical insights on experimental design.
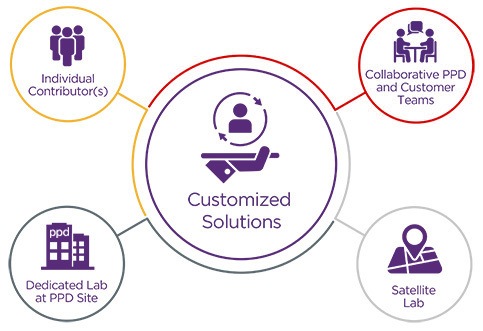
Functional Service Partnerships (FSP): Our team is one with your team
We offer highly customized testing programs designed specifically to your organization and its unique requirements. Our tailored solutions feature extensive testing experience/expertise, and our industry-leading labs enable us to successfully plan, manage and execute your next program no matter where, when or how you need it.
PPD Laboratory Services’ FSP
Related resources
Learn more about how we can help with your needs.
Learn more about PPD Laboratory services GMP lab
To request a proposal or contact your local business development representative, please complete the form below.




