
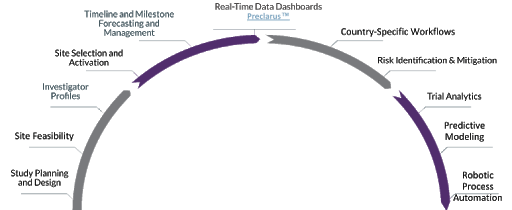
Leveraging Technology to Optimize Trials
Our technology informs, analyzes, automates, simplifies and accelerates your clinical trials
The enormity of conducting global clinical trials can overwhelm the most meticulous planning and design — stretching timelines, exceeding costs and compromising data integrity.
The PPD™ clinical research business of Thermo Fisher Scientific partners with industry leaders in big data, workflow management and artificial intelligence to streamline and simplify complex, multilayered clinical trials and to mitigate risks, paving a clear path to commercial success.
We employ predictive modeling, machine learning and other analytics to:
- Enhance study design
- Optimize country/site selection
- Accelerate site activation
- Speed recruitment timelines
- Predict and mitigate risks
- Automate standard tasks
Select platform automates site identification and feasibility
Our partnership with Oracle goBalto uses cloud solutions to streamline and automate site selection and activation of our top-performing sites, delivering 30% quantifiable reduction in study startup cycle times.
- Identifies sites with the required capabilities, experience, highest enrollment and leading startup cycle-times
- Manages outreach to sites, including CDA collection, site surveys and site tiering
- Facilitates dynamic management of investigator and institution profiles
- Simplifies feasibility via online surveys to gauge study-specific needs, investigator interest, local patient population and site capacity
Activate platform supports site activation and startup:
- Maps out startup and activation processes step by step in 60+ countries
- Establishes workflows per country-specific regulations and investigator requirements to achieve rapid regulatory approval for site activation
- Integrates with CTMS, central IRB, eTMF and IMS for single point of access to all data
Benefits:
- Provides a holistic view of site and investigator experience, performance and capacity by aggregating our data and external data
- Speeds cycle times for site selection and feasibility
- Minimizes burden of multiple, lengthy site surveys by capturing external data sources and recalling historic site survey data
- Enables more structured country-level feedback on site selection process
- Standardizes scoring and analytics to enable more consistent, data-driven decisions
- Fast-tracks prioritized sites with different workflows and surveys
- Proactively identifies and manages risks by providing greater visibility into study, country and site status
Artificial intelligence drives powerful outputs
Our partnership with Medidata harnesses its artificial intelligence and machine learning capabilities to inform your study design, operationalize your study plan and mitigate risks with practical solutions.
Medidata’s predictive analytics suite of tools:
- Guides your study design and country/site selection
- Forecasts and manages study timelines and key milestones
- Models your study’s trajectory to predict pain points
- Identifies and helps mitigate potential risks
Benefits:
- Speeds activation and recruitment timelines
- Reduces low and non-enrolling sites
- Helps minimize study costs
- Automates standard tasks
Unprecedented access to study data in real time
Enables quick, efficient planning and rapid resolution of issues.
Preclarus™ is our award-winning portfolio of technology solutions that analyzes, consolidates and standardizes clinical trial data from multiple sources in a real-time dashboard for instant access and simplified viewing. Users can access the totality of their data in a single location while eliminating static database reports, manual calculations and ad hoc reporting
Benefits:
- Real-time delivery of clinical and laboratory data
- Dynamic analytics and visualization
- Scalable and repeatable capabilities
- Enterprise or centralized data storage