Early Phase Clinical Trials
Navigating early phase clinical trials
Phase I and Ib trials represent the first practical tests of your compound’s clinical relevance and commercial viability. They are the culmination of years or even decades of research. Moreover, they set the stage for subsequent studies that will ultimately determine your compound’s efficacy, safety and positive impact on patients’ lives.
Maximizing success in early phase development requires a highly coordinated effort to anticipate potential challenges, recruit the right patients, engage top investigators and design a trial that will build a platform for later-stage success.
The dedicated team of the PPD™ clinical research business of Thermo Fisher Scientific global experts in early development and clinical pharmacology performs a critical assessment of your compound’s unique attributes to identify areas of strength and potential weakness that could impact the outcome of your current and subsequent trials. The team develops an integrated, early-phase program that leverages our facilities, site network, operational expertise and development experience to execute your trial in patients and/or healthy volunteers. Each step of the process is conducted with an eye toward supporting later-phase programs and regulatory submissions.
Ensuring patient diversity in clinical trials
Inclusive research solutions curated to meet your needs at every phase of drug development.
Helping our clients navigate their Phase I-IB studies
Our early development services keep an eye toward supporting your later-phase programs.
Phase I studies conducted in the past five years.
Households in our patient database.
Our early phase development and clinical pharmacology solutions
Our streamlined services help establish proof-of-concept by reducing time and cost from your development timeline.
Early phase CRO services

Phase I clinical trial capabilities
First-in-human studies are demanding and multidimensional, requiring a finely tuned balance between scientific rigor, patient safety, regulatory requirements and market access strategies.
Orchestrating these disparate elements requires an experienced and dedicated team of experts who collaborates to inform, advise and execute your Phase I studies. Our dedicated Phase I professionals customize solutions to fit your unique compound, business model and study objectives.
Focus on complex study design
We understand that early phase development in complex studies requires a highly coordinated effort to anticipate challenges, recruit the right subjects, engage experienced investigators and design a trial that will establish a platform for later-stage clinical success.
Our early development team performs complex studies through our global network of sites. With over three decades of experience conducting Phase 1 trials, our teams bring a unique understanding of high-quality performance, and we work with our sites to ensure conduct and data meet an exacting standard.


Flexible solutions customized to fit your phase I study needs
Thermo Fisher Scientific’s PPD clinical research business provides comprehensive and flexible pharmacokinetic (PK) and pharmacodynamic (PD) support of Phase I-IV clinical trials. We offer clinical pharmacology expertise as part of an integrated drug development program or as a discrete stand-alone service with clinical, scientific and regulatory experience in a variety of areas.
- Translational medicine, including toxicokinetics (TK)
- Clinical pharmacology program and protocol development and support
- Analysis and interpretation of PK and PK/PD data
- Scientific and medical expertise aligned to each client’s pipeline
- Comprehensive access to patients, healthy volunteers and special populations
- Experience in design and execution of both adaptive and traditional early phase studies
- A fit-for-purpose, flexible operating model
Early clinical development global capabilities
The partner you select for your early phase studies is critical in helping you achieve your overall development timeline efficiently and within budget. You need a contract research organization (CRO) that has an established network of carefully selected sites centered around quality, medical safety and unparalleled expertise.
We understand the importance of selecting the right sites when executing Phase I studies. Each of our global facilities undergo a rigorous selection process including GCP audits and/or pre-selection assessment visits. We have executed master clinical service agreements or agreed to contract language with all our sites.
47 early development sites spanning across 11 countries
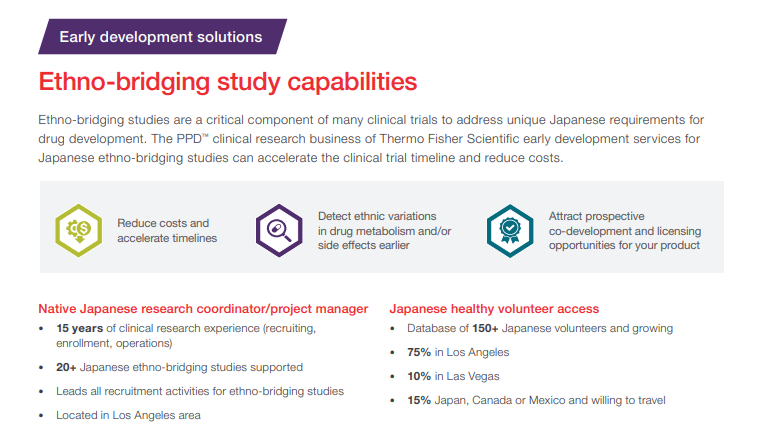
Our global network of Phase I clinical sites offer access to a diverse pool of healthy volunteers and specialty populations across four continents. Many of our 47 strategically located facilities are located near hospitals and universities, allowing us access to hundreds of faculty members in fields such as pulmonology, anesthesiology and neurology. With over 100 studies awarded through our global network in the past five years, our areas of expertise include: renal impairment, hepatic impairment, Japanese Bridging, Chinese Bridging in China and NHV studies.
Supporting indications and specialty populations through a global site network
Indications include:
- Dermatological conditions
- Digestive
- ENT
- Genitourinary
- Hepatic/renal
- Immune/inflammation
- Infectious diseases
- Cardiovascular/metabolic
- Musculoskeletal
- Neurological
- Respiratory
- Sexual disorders
Specialty populations include:
- Elderly
- Ethnic specialty populations
- Pediatrics
- Postmenopausal females
- Smokers
- Hepatic/renal impaired
Broad early development experience
Our three U.S.-based clinical research units are broadly experienced in special procedures with a dedicated focus on complex studies, first-in-human studies and neurodegenerative diseases. With over 30 years of experience in executing Phase I studies through our CRUs, our facilities are host to cutting-edge diagnostic tools, technology and registered medical experts.

Early development services: ADME study capabilities
Our early development services can perform absorption, distribution, metabolism and excretion (ADME) studies to understand how a drug is processed in the human body to assess safety and efficacy.