Choosing Decentralized Clinical Trials
The rise of DCTs
The COVID-19 pandemic has challenged the viability of traditional clinical trials around the world. The use of decentralized clinical trial (DCT) options was on the rise before the pandemic, but the industry’s growing need to keep research moving forward and ensure the safety of clinicians and patients made the need for remote capabilities more important than ever. With new efficiencies produced by telemedicine, remote monitoring, direct-to-patient shipments, devices and more, these new options will remain an important attribute of clinical trials as we enter the digital age of research.
of respondents expect to run a hybrid/decentralized trial in 2022 (vs. 59% in 2021).
Why choose DCTs
As the clinical landscape continues to evolve, DCTs present opportunities to emphasize convenience, safety and flexibility, while continuing to pursue quality data and valuable research. Advantages include:
Environmental sustainability
The PPD™ clinical research business of Thermo Fisher Scientific is dedicated to creating environmental innovations to better predict, measure and identify strategies to reduce the greenhouse gas emissions on clinical trials. We are partnering with stakeholders across the clinical trial spectrum to better understand how we can digitize, decentralize and modernize clinical research to make it more environmentally friendly.
Learn more about how we are creating a healthy planet to support healthy patients
Foster a sustainable future in clinical trials through decentralized trial models


2022 Decentralized Clinical Trial Sites survey
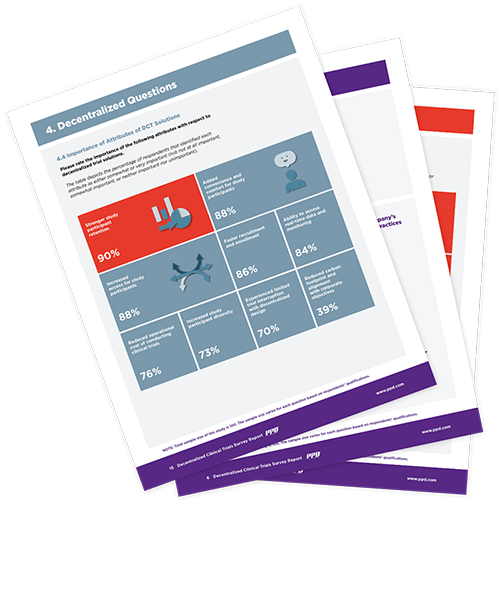
Our 2022 Decentralized Clinical Trial Sites survey offers a look into a shifting and expanding market, allowing us to see where DCTs are headed and better understand site needs. We surveyed high-level clinical trial site staff to provide invaluable industry insights. As the adoption of DCTs increases, it is essential to anticipate market needs, challenges and impacts.
2021-2022 Decentralized Clinical Trials Industry Report
In 2021, after pandemic-fueled growth in decentralized clinical trials, we mined industry opinions on the likely future composition of clinical trial operations. The analysis aimed to determine how hybrid and fully decentralized models will be used alongside traditional constructs in efforts to increase efficiency, improve outcomes, and speed the progress of life-saving therapeutics.
Access the data and insights on how the industry is pivoting to tackle clinical trials during the pandemic and beyond.

Interest in Decentralized Trials Rising in Asia Pacific
Even though cultural factors currently impact the acceptance rate of decentralized clinical trial (DCT) models in the Asia-Pacific (APAC) region, most countries in the APAC region are at the forefront of advanced technologies, and future widespread adoption of DCTs is anticipated.
Learn about how the current landscape of DCTs in the APAC region and how we can support future trials.
What Are Some of the Challenges Sites Experience When Implementing DCTs?
Decentralized clinical trials (DCTs) span a broad spectrum of designs and iterations, the bulk of which are currently hybrid trials that include some aspects of both digitally enabled and decentralized approaches. While sites may experience some challenges, sponsors and clinical research organizations (CROs) can take steps to support site staff. Working with a DCT vendor — one who is reliable and consistent, has a proven track record and delivers high quality — is crucial for success.
Learn about how our white-glove service supports sites on their DCT journey. Access the article below.

Exploring Challenges and Considerations for Operationalizing DCTs in the European Union
While conducting decentralized clinical trials (DCTs) in Europe involves navigating a complex landscape of regulations, national requirements, and languages, they also offer unique trial opportunities. Through careful, informed design — and support from an experienced provider — the European DCT landscape can help bring new therapies to market.
Learn about how we support European DCTs and clinical trial sponsors with a holistic and comprehensive approach. Access the article below.
Evolving Solutions to Optimize Clinical Trial Decentralization
As the adoption of decentralized and hybrid clinical trials continues to grow, trial designers and researchers are increasingly adopting novel solutions and new technologies to decrease patient burden and increase levels of successful adherence.
See how we are removing the rigidity associated with traditional trial design and focusing on innovation and patient centricity. Access the article below.


We were named a leader in digital transformation by ISG
Information Services Group (ISG) recognized us in its 2024 ISG Provider Lens™ Life Sciences Digital Services Global Report that assesses the use of technology innovation to assist in drug development. The findings recognize us as an ISG Provider Lens Leader for clinical development digital transformation services and patient engagement digital transformation.
2020 Clinical Trial Insights Report
To guide organizations through a transformative time in clinical research, we held a number of webinars exploring the accelerated pace of adoption for novel approaches and innovations impacting clinical research, with our experts offering strategies and considerations to support the rapid changes happening within clinical trial design and operations.

Examining Implementation Timelines For Decentralized Clinical Trials (Dcts)
While DCTs can still enable trials that would otherwise be challenging during the pandemic, many of the potential efficiencies — particularly rapid implementation — can remain out of reach. We have DCT strategies built into the protocol from the start, with custom features and functions instead of a one-size-fits-all approach.
Learn more about implementation timelines for decentralized trials.


Building Decentralized Clinical Trials for Patients and Clinicians
The increased implementation of decentralized clinical trials means the industry now has a considerable body of evidence and experience to test the strengths of digital clinical trial models. With the growing amount of data comes the ability to assess how to make trials more effective and efficient, including a closer look at qualitative sponsor, site and patient feedback. To gain further insight into this transformation, we explored the experiences that key stakeholders have had when approaching decentralized clinical trials.
Protecting clinical research and patients through decentralization
Today’s biopharma and biotech organizations are increasingly opting for digitally enabled trial designs over traditional solutions. Digital and Decentralized Solutions works closely with life science leaders from around the world to navigate evolving trends in drug development, including the design and execution of bespoke decentralized clinical trials.

Digital innovation and implementation
How can our suite of digital capabilities and solutions help you reach more patients?