
The Top Seven Things to Understand About Data Monitoring Committees

In this blog post, PPD’s Robert King, global senior director of functional service partnerships (FSP) – biometrics, Simon Weeden, director of biostatistics, and Lindsey Lian, senior director of biostats and programming, discuss best practices that can be applied to data monitoring committees.
1. What is a data monitoring committee (DMC)?
The FDA defines a data monitoring committee as “a group of individuals with pertinent expertise that reviews, on a regular basis, accumulating data from one or more ongoing clinical trials. The DMC advises the sponsor regarding the continuing safety of trial subjects and those yet to be recruited to the trial, as well as the continuing validity and scientific merit of the trial.” DMCs are independent of the study sponsor to ensure there is no bias in decision making. They can also be known as data and safety monitoring boards (DSMBs), data and safety monitoring committees (DSMCs) or independent data monitoring committees (IDMCs).
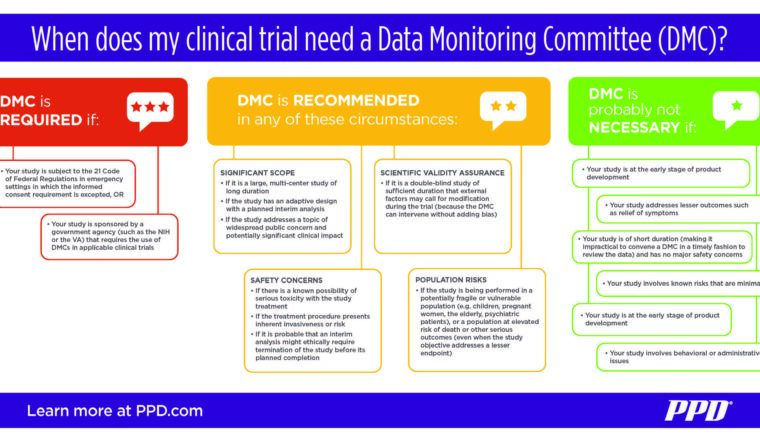
2. When is a DMC recommended for a clinical trial?
Not every study needs a DMC and there are a few strict guidelines to dictate exactly when a DMC is required. This leaves some amount of gray area in deciding the DMC question. Generally speaking, a study that involves significant safety concerns, risks or complexity should consider the use of a DMC. Some examples include:
- A large, multicenter study of long duration, or one with an adaptive design and planned interim analysis
- A study involving a known possibility of serious toxicity or an invasive treatment procedure
- A study being performed in a potentially fragile or vulnerable population (such as children, pregnant women, the elderly or the terminally ill)
- A double-blind study with the probability of modifications being necessitated by external factors during the trial (in which event the DMC can intervene without adding bias)
Current U.S. Food and Drug Administration (FDA) guidance mandates the use of DMCs in only one specific instance: under 21 CFR 50.24(a)(7)(iv) for research studies in emergency settings in which an exception to the requirement for informed consent requirement has been approved.
Beyond the FDA, some U.S. government agencies require DMCs in certain clinical trials they sponsor. This includes the National Institutes of Health and the Department of Veterans Affairs.
The European Medicines Agency (EMA) guidance does not set out requirements for the mandatory use of a DMC, but their recommended use is subject to the considerations above. For studies with adaptive designs leading to design modification(s), EMA also recommends that the sponsor seeks regulatory advice on the proposed modification(s).
3. When is a DMC not necessary for a clinical trial?
Most short-term, smaller studies involving low risks do not need a DMC. This would generally include early-stage development, trials of short duration without critical safety concerns and studies designed to address less-essential outcomes, such as relief of symptoms.
4. Who should serve as members of a DMC?
A DMC needs a minimum of three members. These should include a medical doctor who specializes in the therapeutic area or condition that is the focus of the study; an additional medical doctor experienced in clinical trial conduct and/or the focus of the study; and a biostatistician with knowledge of sequential analysis of clinical trial data. DMC members can be drawn from academia and medical establishments as well as the pharmaceutical industry. Larger studies may need more DMC members for a broader range of expertise, and in many cases a second, non-voting biostatistician will serve as the committee’s unblinded support. All DMC members must be vetted to avoid potential conflicts of interest or direct personal involvement in the study.
5. What is a DMC charter?
Every DMC should work to a detailed charter that serves as a governing document of standard operating procedures. Like study protocols, charters document the full catalog of methods and procedures that were established from the start, to guard against concerns of bias or impropriety over the course of the trial. The charter may be written by either the sponsor or the DMC, with the other party reviewing and approving, and should be prepared before patient recruitment commences. Its contents will circumscribe the particulars of committee meetings, access to and presentation of interim data, how reports are given to the DMC, and other relevant matters.
6. What are the responsibilities of a DMC?
As independent and objective reviewers, the DMC is responsible for monitoring interim study results for effectiveness, safety and proper study conduct, as well as giving consideration to relevant external data. Importantly, the committee recommends to the sponsor whether the study should continue as designed or not. Based on their assessments, the DMC may recommend modifications to the protocol, a temporary suspension of enrollment or intervention, or termination of the study. Above all, the safety of patients is of utmost concern to the DMC, and its duties are of critical necessity to evaluate a study’s accumulating evidence for safety and efficacy.
7. Best practices for supporting DMCs within PPD
At PPD, our experience and expertise in DMCs has led to us establishing best practices to ensure patient safety is protected. These best practices achieve DMC objectives whilst efficiently managing investments, timely responses to requests and better management of time and resources — benefiting both the sponsor and the patient’s safety.
In particular, PPD routinely manages the blinded and unblinded activities for whole studies to maximise efficiencies without compromising the study’s blinding status. Some of our best practices include:
- Ensuring appropriate firewalls are in place for confidential DMC activities such as generating unblinded analyses and providing these to DMC members. The integrity of the clinical trial depends on appropriate safeguards being in place to protect the sponsor and participating staff from receiving unblinded information.
- Discussing planned analyses with the DMC and sponsor at the outset of the study. Agreement on the collection and presentation of key data points upfront will ensure the DMC receives the data it requires for review and will hopefully minimize inefficient and costly rework.
- Agreeing to a data strategy including a data cleaning process to meet the DMC and sponsor’s expectations. Without clean, complete and current data, DMCs cannot make the right decisions. For example, for DMC safety reviews, time is of the essence and they will require as close to real-time data as possible to ensure the safety of the participants.
- Establishing standard operating procedures and best practices to ensure appropriate access and distribution of confidential analysis results, as well as the proper communication pathway among the DMC, the sponsor, the unblinding statistical analysis team, and the blinded study team. Doing so will minimize the risk of accidental study team unblinding, therefore ensure the study scientific integrity.
- Placing lead statisticians with the right skill set to successfully work with DMC members to ensure seamless meeting conduct and appropriate discussion. Key qualities include relevant statistical expertise, good communication skills, understanding of the study design, objectives and treatments, the ability to understand and satisfy the DMC’s requests while best using time and resources, and being able to communicate with the study team while maintaining the study treatment confidentiality.
- A solid plan for supporting all DMC logistics (member selection, contracts, meeting organization, minutes, etc.) is crucial. This may seem trivial, but logistical failures can have a devastating impact on the DMC’s and sponsor’s confidence in the process, as well as on timelines and budgets. PPD combines our SponsorView technology with a dedicated team to manage these logistics.
- Develop a contracting approach that ensures DMC independence so that there is no real or perceived influence on the membership. With a budgeting structure in place, it will allow an acceptable level of flexibility to accommodate the evolving needs of the committee without impeding their focus on protecting patient safety and trial integrity as the trial progresses.
Learn more about PPD’s capabilities
To continue the conversation