
Five Challenges and Solutions in the mRNA Vaccine Manufacturing Process
As mRNA (messenger RNA) technology expands, so do challenges in manufacturing mRNA vaccines. Learn the five major barriers developers face — and solutions for navigating these obstacles.

Applications of mRNA technology are only continuing to expand. Researchers are revolutionizing medicine by leveraging the power of mRNA technology to develop preventative vaccines and therapeutic treatments for emerging and previously untreatable diseases and indications.
Developers entering the mRNA vaccine and therapeutic space, as well as more experienced biotechnology and pharmaceutical organizations, may face daunting manufacturing challenges. Working with multiple vendors throughout the mRNA manufacturing process can complicate development, with the potential for operations to come across as an uncoordinated “relay race” that risks miscommunication and delays.
Partnering with the PPD™ clinical research business of Thermo Fisher Scientific positions developers for success from start to finish by combining our clinical, operational and regulatory expertise with Thermo Fisher’s extensive product and service capabilities.
As a provider of CRO services, Thermo Fisher Scientific’s PPD clinical research business is one of the few in the industry with a dedicated vaccine therapeutic unit, delivering resources with specific vaccine and infectious disease expertise. We have more than 30 years of vaccine experience, from pre-clinical to post-licensure, and more experience supporting FDA-approved vaccines than any other contract research organization (CRO). For two consecutive years, we were named “Best Contract Research Organization” at the 2021 and 2022 World Vaccine Congresses.
Our customers are overcoming common hurdles the industry faces in mRNA vaccine manufacturing — and are readily able to streamline operations, speed development, and contain study, manufacturing and storage costs.
In this blog, we’ll review some of the specific hurdles developers face regarding manufacturing of mRNA vaccines — and ways to overcome them — including:
- Uncoordinated and inefficient processes
- Limited accessibility of Good Manufacturing Practice (GMP)-grade raw materials for mRNA manufacturing
- Complexity of manufacturing steps
- Lack of experience and risk of error in mRNA production, product analysis and formulation
- Fill-finish, storage and logistics limitations
Keep reading to learn more about these challenges in mRNA manufacturing and ways to overcome them.
Challenge 1: Uncoordinated and inefficient manufacturing processes
The mRNA manufacturing process is made up of discrete, specialized steps. In theory, biotechnology developers could outsource every step to a different manufacturing partner, each with different pricing, capacities and specialized knowledge. However, this “divide-and-conquer” strategy requires developers to coordinate between multiple vendors, which can result in delays, duplicative work and bureaucratic headaches. These issues make the process inefficient and difficult for smaller, emerging companies that lack the bandwidth to support these logistics.
Solution: Relying on one partner rather than separate vendors cuts through red tape and makes expenditures and logistics more efficient. To solve these coordination problems, we offer an end-to-end portfolio of PPD clinical development solutions for companies or agencies of any size. When your chosen partner is invested in quality throughout the entire process, the collaboration leads to shared program goals and ownership.
Smaller, emerging companies entering the mRNA space can streamline their operations with Thermo Fisher Scientific’s Quick to Care™ program. This solution offers an integrated, bundled approach to services by providing plasmid DNA, mRNA manufacturing, lipid nanoparticle (LNP) formulation and fill-finish services all at one site. Thermo Fisher Scientific’s solutions also introduce efficiencies for self-manufacturing by larger, more established drug makers by providing process development insights, access to clinical research site networks and more.
Challenge 2: Limited accessibility of GMP-grade raw materials for mRNA vaccine manufacturing
Any disruption in raw material supply will delay production, create bottlenecks and long lead times, and leave investors dissatisfied. Unfortunately, supply chain reliability has been a rising concern in recent years, with existing vulnerabilities and pain points exacerbated by the COVID-19 pandemic.
Solution: To prevent material shortages and shipment delays, researchers, biotech and pharmaceutical developers need access to an established, diversified global supply chain. That way, even if one supplier fails, another source can step up with the necessary reagents, equipment and other materials. Thermo Fisher Scientific and its PPD clinical research business maintain business relationships with an extensive network of global suppliers, giving manufacturing partners access to a supply chain that is reliable, even in volatile times.
To further ensure a secure and stable supply of critical raw materials, Thermo Fisher Scientific has dramatically expanded its manufacturing capabilities and capacity to produce nucleotides and enzymes (Thermo Fisher Scientific TheraPure™ GMP products, figure x). These materials have been successfully used in commercial mRNA therapeutics and vaccines and can be delivered at the GMP-grade quality level, speed and scale needed to support very aggressive drug development timelines.
Our offerings include:
- Technical partnership: Tools and technical expertise to enable stability in process development, scale-up and commercial manufacturing
- Quality at scale: Seamless progression from preclinical development to commercial manufacturing and regulatory filing
- Proven products: Standard products used in multiple clinical and commercial mRNA vaccines and therapeutics
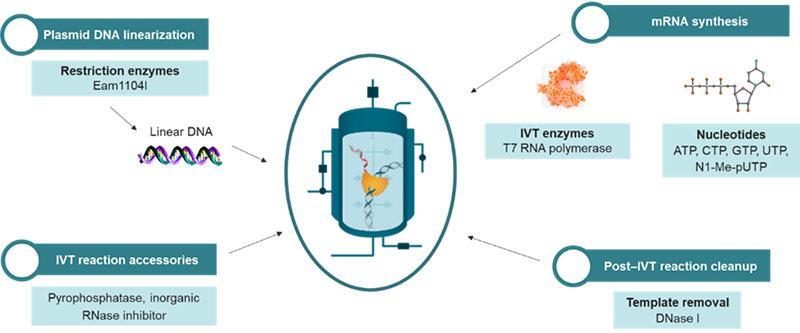
Challenge 3: Complexity of the manufacturing process
One of the reasons mRNA is such a promising platform for vaccines and therapeutic development is that, in principle, the in vitro reaction needed to generate and amplify the mRNA transcripts is relatively simple and rapid. However, in practice, synthesis and subsequent purification processes needed to obtain uncontaminated mRNA transcripts are anything but simple. There’s no shortcut when it comes to bringing this complex and time-intensive step to completion.
Solution: Clinical research and development partners that understand every precise detail of a customer’s request. Any errors, or DNA contamination, can lead to massive losses of time, effort and monetary investment. More than 2,000 PPD staff members have specialized vaccine experience, supporting more than 220 vaccine studies in the past five years. Paired with our production processes (figure x), QC methods optimization, state-of-the-art contract manufacturing and development facilities, and technological capabilities, this experience ensures that each step is completed with technical rigor.
Challenge 4: Lack of manufacturing process experience and risk of error in mRNA production, product analysis and formulation
Producing mRNA on a large scale for commercial use can create a significant technical and process development challenge. mRNA is produced biosynthetically and requires a significant amount of expertise to understand and control the variables in the multistep production processes (plasmid DNA template production, in vitro transcription, mRNA purification, etc.). Because the use of mRNA technology to produce therapeutics and vaccines is very new, many companies have little to no experience with producing mRNA at scale.
By the time an mRNA product has reached the point of analysis and formulation, developers have made a considerable investment of time and money. Because batch loss at this point would be detrimental, it is critical to have personnel with specialized expertise who can perform analyses efficiently without the risk of error and ensure that products can proceed to formulation and fill-finish.
Solution: Experience in the mRNA manufacturing process, from plasmid DNA to fill-finish, and a trusted, proven network. The PPD clinical research business of Thermo Fisher Scientific has more than 30 years of experience in developing and testing vaccine assays, along with a wide network of dedicated personnel who can meet the needs of developers in this important step thanks to their strong expertise in statistics, technical processes and quality assurance. Additionally, our labs are equipped with advanced technologies that can provide precise, accurate and reproducible measurements and high-quality data for confident assessments, and can also support drug product pre-formulation and formulation development programs. Thermo Fisher Scientific has an extensive worldwide network of field-based technical support and product specialists who cover all aspects of the mRNA production process and have worked with several partners to successfully bring mRNA-based drugs to market.
Challenge 5: Fill-finish, storage and logistics limitations
mRNA is more unstable than DNA and must be handled at extremely low temperatures — and, as a result, manufacturers must have access to reliable cold chain storage, which can be expensive to install if it is not already in place. Furthermore, a manufacturing partner’s choice of packaging can create additional storage issues. With many years of experience in the mRNA space, we have demonstrated that we have the resources, capabilities and expertise to meet specific demands, such as cold chain storage and handling.
Once produced, developers must ensure that their well-formulated product successfully reaches every corner of a distribution network. mRNA vaccines and therapeutics are vulnerable to mishandling, accidents and improper storage.
Solution: End-to-end transportation services. To ensure expert coordination of logistics, our customers can take advantage of total transportation management as one of the value-added services that are part of Thermo Fisher Scientific’s Quick to Care program. Developers ship products with confidence using our global network of qualified carriers and interim storage providers. Partners can also reduce risk and realize cost savings through our continuous monitoring and proactive risk mitigation throughout the supply chain, along with customs and regulatory guidance.
After initial manufacturing, distribution and clinical successes, many biopharmaceutical developers find themselves needing to dramatically scale up their operations to meet demand or satisfy regulatory testing. However, making the necessary process changes while operating with limited existing infrastructure can create logistical challenges. Fortunately, Thermo Fisher Scientific and its PPD clinical research business have expertise in the mRNA manufacturing process and a global network of extensive, GMP-compliant facilities that enable efficient scale-up.
The right research partner: From plasmid DNA to fill-finish
Partnering with the right regulatory, operations and clinical expertise, mRNA vaccines and therapeutics have the potential to be designed and developed with greater efficiency and at a reduced cost.
mRNA vaccines show strong potential for use in developing vaccines for a wide range of indications, and COVID-19 certainly accelerated interest in them. It will be essential for government and public health leaders, biotech, and pharmaceutical organizations to partner with a CRO that has a track record of success with the mRNA manufacturing process – thereby demonstrating the ability to support expanded mRNA manufacturing as the field continues to grow.
Thermo Fisher Scientific and its PPD clinical research business are uniquely positioned to support mRNA manufacturing by providing a complementary end-to-end portfolio of products and services – from plasmid DNA to fill-finish – that uniquely exceeds the capabilities of any other supplier, CRO or contract development and manufacturing company (CDMO).
Additional contributors:
Thant Zhu, sr. market development manager, Thermo Fisher
Darwin Asa, market development manager, Thermo Fisher
In the past five years, Thermo Fisher Scientific’s PPD clinical research business has completed more than 220 vaccine trials, including more than 68 COVID-19 vaccine studies and 100 mega-trials with more than 200,000 participants. We are one of only two providers of CRO services to execute a full clinical development program for a COVID-19 mRNA vaccine that has received full approval by the U.S. Food and Drug Administration. Thermo Fisher Scientific’s capabilities in mRNA manufacturing and storage provide our clients with an advantage and additional benefits to our end-to-end capabilities.