Top 5 Challenges and Opportunities Drug Developers Face in Executing Clinical Trials
Discover key barriers and possibilities drug developers share in advancing clinical trials.

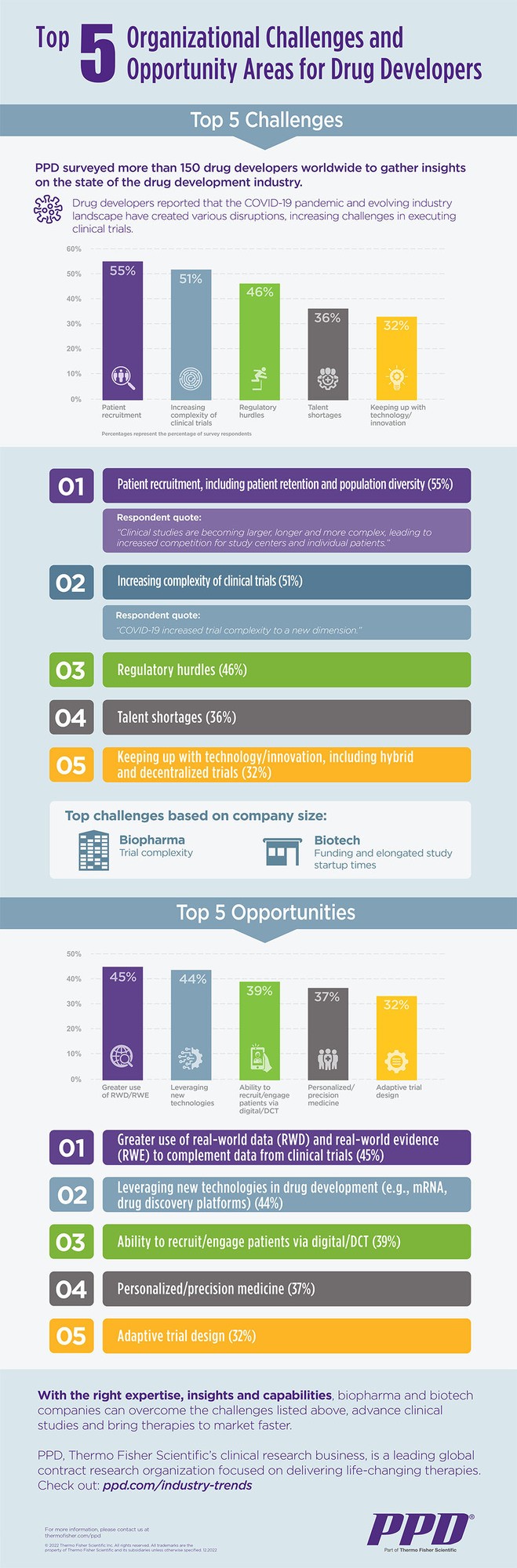
PPD, Thermo Fisher Scientific’s clinical research business, surveyed more than 150 decision-making leaders at pharmaceutical and biotech companies around the globe to collect key insights on the state of the evolving drug development industry. Drug developers shared the disruptive impact of the COVID-19 pandemic and ever-changing industry landscape, which resulted in increased challenges in clinical trial execution.
Respondents shared sentiments around current challenges within their organizations and the broader industry. The top challenge varied depending on company size: for biopharma companies, the top challenge was trial complexity, while for biotech companies, the top challenges were funding and elongated study startup times.
Key takeaways include:
- Patient recruitment, including patient retention and population diversity, tops the list of challenges that 55% of respondents face. One respondent shared that clinical studies are becoming larger, longer and more complex, leading to increased competition for study centers and individual patients.
- Increasing complexity of clinical trials followed as the second-most reported challenge. One respondent shared that they believe COVID-19 led to the increase in trial complexity.
- Regulatory hurdles, talent shortages, and keeping up with technology/innovation, including hybrid and decentralized trials, rounded out the list of top reported challenges.
Amid the challenges, drug developers also noted several areas of future opportunity:
- The largest proportion (45%) of survey participants consider greater use of real-world data (RWD) and real-world evidence (RWE) to complement data from clinical trials as the greatest opportunity area.
- The opportunity to leverage new technologies in drug development (e.g., mRNA, drug discovery platforms) directly followed, with 44% of respondents reporting it as the greatest future prospect.
- The ability to recruit/engage patients via digital/decentralized trials, personalized/precision medicine and adaptive trial design rounded out the list of the top 5 opportunities.
Biopharma and biotech companies can overcome the challenges noted by partnering with a clinical research organization that integrates data usage, leverages new technologies, and embraces innovative approaches to advance clinical trials and quickly bring therapies to market.