Cell and Gene Therapy Lesson 5: Lab Concierge – Extreme Focus on the Rare Disease Patient and Precious Study Sample Logistics Through an Innovative Lab Concierge Program

Patrick Bennett, VP lab operations, PPD Laboratories biomarker lab and Janine McKnight, associate director project management, PPD Laboratories biomarker lab, explain a concierge program focused on rare disease patients.
Development of cell and gene therapies has increased by approximately 800% year over year, outpacing all other therapies by a staggering amount. 1,2,3,4 However, in comparison to traditional clinical studies, cell and gene therapy studies are extremely unique and are currently focused on the therapeutic areas of rare diseases and oncology. A key attribute for these studies and their associated study samples includes the unknown timing and geographic location of an enrolled patient. Therefore, the challenge becomes the oversight of these samples from point of collection to the various points of testing meeting the specific time and shipping condition requirements for very few samples. PPD Laboratories offers an innovative lab concierge program that puts extreme focus on rare disease patients and the security of the precious samples associated with them. During the current COVID-19 pandemic, this service has become even more critical. It has also become extremely important to support clinical trials for SARS-CoV-2 vaccines and various COVID-19 treatments.
“From cell collection, sample processing, and infusion to post-infusion care, CAR-T cell therapy requires the close collaboration of many departments within an organization. Building a dedicated multidisciplinary cellular therapy team and creating practice guidelines, efficient workflows, and clear communication are vital to ensure safe administration of CAR T cell therapy,” said Dr. Panteli Theocharous, VP cell and gene therapy business strategy for PPD.
Frequently, patients in the rare disease and oncology therapeutic areas are neonatal or pediatric patients. Zynteglo from Bluebird Bio was recently approved in Europe for a rare condition called beta thalassemia in patients 12 years and older4. Zolgensma was also recently approved for pediatric patients under 2 years of age to treat spinal muscular atrophy (SMA). Identifying a patient with these rare conditions and enrolling them into a study is challenging. The search will be global in nature with very low visibility into where and when a patient will be qualified for the study, meaning that each sample is extremely valuable. Any loss of sample or the inability to test the sample for key biomarkers can be devastating to the patient, their family, the drug developer and other patients with this disease.
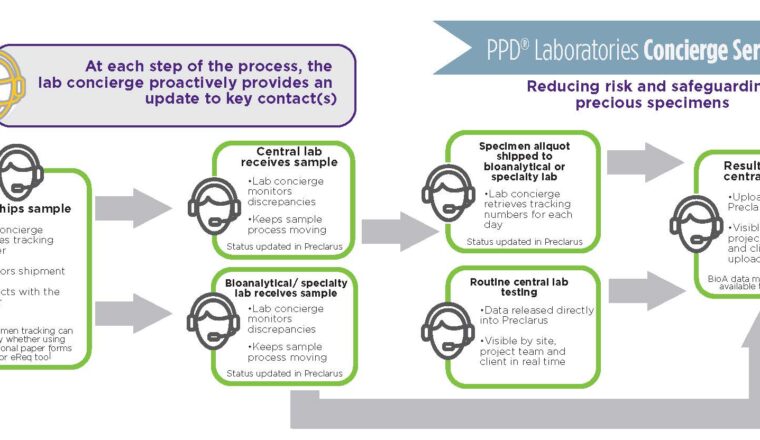
Once a patient is identified and enters the study, the issues surrounding the collected sample logistics become apparent. Therefore, a strong system of communication and tracking is vital. Storage conditions, duration, methods and tracking of the shipping route of the transported samples are crucial and require advanced planning. The collected sample must then be distributed to the testing laboratory under the correct storage conditions. The labs must also be aware of when the samples are arriving due to sensitive time frames for stability. This is common with flow cytometry-based methods.
“We receive human whole blood and bone marrow samples from multiple sites for flow cytometry analysis, including CAR-T cell enumeration. These samples need to be analyzed within 48-72 hour after collection due short stability of the cellular markers. Our concierge personnel track these samples and are in constant communication with the laboratory team, including weekends and holidays to make sure the samples are received and analyzed within the stability period,” said Vellalore “K” Kakkanaiah, director of biomarker services for PPD Laboratories.
Furthermore, the required testing may require multiple laboratories in different parts of the world depending on the availability of the testing method.
Because of the rare occurrence of the diseases being treated, patient identification and recruitment creates significant challenges. The unknown timing and geographic location of study samples results in complex logistics to perform necessary lab testing. A wholistic sample logistics strategy must be created prior to the protocol approval. This strategy includes identifying the types and sample volumes needed, associated sample collection kit requirements, the organization and frequency of shipments of kits, sample storage and transport conditions, necessary import/export permits, and turnaround timing for lab testing.
PPD Laboratories’ innovative lab concierge program is not a traditional CRO process, it is an ultra-attentive, specialized, individualized project management team that specifically focuses on sample management and logistics to eliminate the above challenges. Lab concierge streamlines communications with sponsors, central lab project managers, sites, shippers, data management teams, PPD Laboratories’ bioanalytical lab and third-party vendors. This cross-functional communication is necessary to ensure sample receipt, testing, initiation and coordination of data transfers.
The concierge ensures there is proper logistics coverage for each study 365 days a year. All team members remain current on the status of each patients’ samples through custom proprietary tracking software that can be designed for each study. The concierge team is adept at delivering creative solutions for tracking samples, including automation and custom reporting that can be triggered using a mobile device. These reports can also be customized based on rules and triggers provided by the sponsor. They allow early notification and continuous reminders so that critical samples are shipped and tracked in a detailed and real time manner for downstream testing.
Additional resources: