
Advantages and Considerations for Common Regulatory Outsourcing Models

In Pharma Focus Asia, PPD experts detail the common functional service partnership models and compare several key factors of each with case studies.
As the outsourcing of services for drug development becomes more popular across the globe, so does the use of dedicated functional service partnerships.
The five main types of functional service partnership (FSP) models — fixed price, unitized, output- or performance-based, full-time equivalent (FTE)-based and time & materials (T&M) — can provide a wide range of benefits, such as increased efficiency and flexibility.
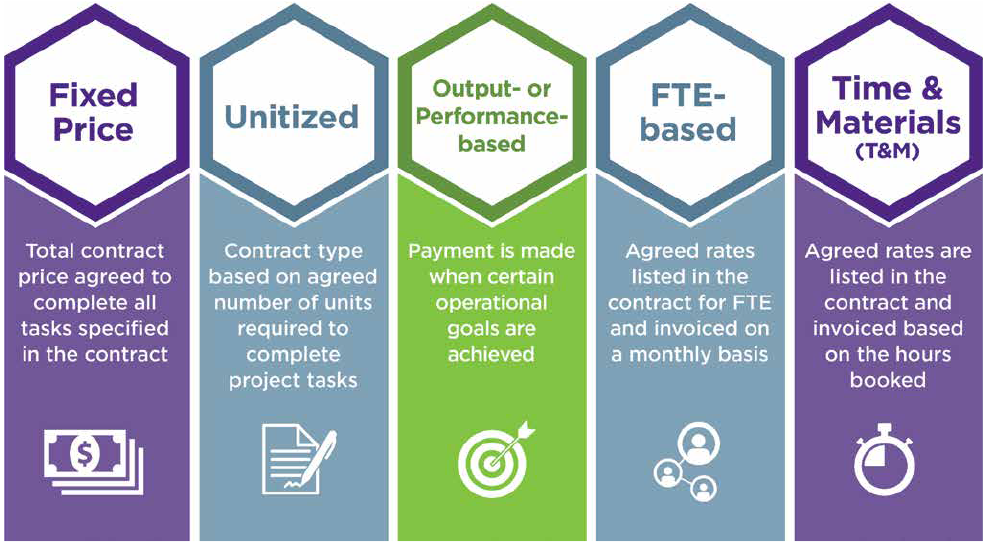
Customers are finding that by bundling high-volume tasks across multiple projects, deploying an FSP model lowers total development costs and reduces operational burden. By leveraging the appropriate FSP model (or models) for their study, customers are more likely to achieve regulatory success while ultimately saving time and money.
FSP models offer the most benefit when both the contract research organization and the customer are clear on the type of work needed and the overall expectations, and they share a commitment to open communication.
Check out this Pharma Focus Asia article authored by four PPD experts to learn more about the five most common functional service partnership models and how the key factors of each compare through specific case study examples.