How to Fast-Track Clinical Research Timelines
Speed is critical at every stage, yet R&D efficiency often remains evasive. Here’s how drug developers can overcome slowdowns to accelerate their studies.

Acceleration in every step of clinical research is critical to pharmaceutical developers. Yet, today’s clinical research sponsors face significant challenges. Despite advances in technology and innovation, pharmaceutical leaders say that trials are increasingly complex, particularly when it comes to patient recruitment and site engagement.
Rather than accepting the persistent delays in clinical research as disruptive, yet unavoidable, we believe they underscore the need for greater efficiency.
To accelerate clinical research timelines, pharmaceutical developers need to tap into solutions that demonstrably streamline drug development from start to finish.
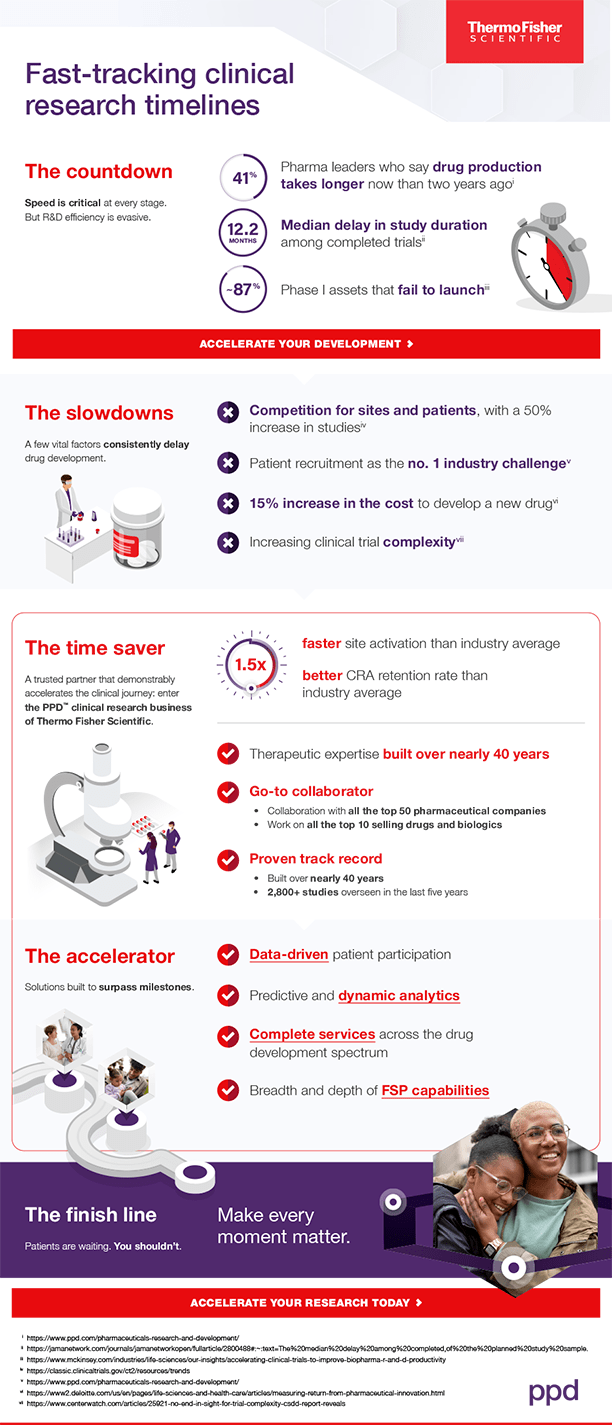
The countdown: Time constraints abound
Our latest pharmaceutical industry survey reveals a concerning trend: Over the past two years, 41% of pharmaceutical leaders have witnessed an increase in the time it takes to bring a drug from first-in-human to market.
These delays financially disrupt the potential of both current and future clinical research. With a median delay of 12.2 months in study duration for completed trials, each year’s setback can risk hundreds of millions in risk-adjusted net present value across a drug developer’s portfolio.
Given that nearly nine out of 10 Phase I assets never make it to launch, it’s clear there’s a pressing need for improvement.
The slowdowns: Common roadblocks impede progress
Pharmaceutical developers face numerous challenges, from fierce competition for sites and patients to soaring development costs and the complexity of studies.
Major hurdles include:
- Clinical trial volume:. Since 2020, registered studies have surged by 50%, intensifying the competition for resources, patients and sites.
- Patient recruitment, cited by 55% of industry professionals as the top obstacle they face today.
- Development costs, which have risen by double digits.
- Extensive number of protocol modifications:. The majority of Phase I, II and III studies encounter at least one substantial amendment, necessitating enrollment suspension, participant reconsent, and internal and regulatory approvals.
These disruptive and costly challenges shouldn’t be accepted as inevitable or simply a part of the process; instead, they underscore the urgent need for a more efficient approach.
The time saver: A trusted partner that demonstrably accelerates the clinical journey
Pharmaceutical developers often seek assistance from partners like us at the PPD clinical research business of Thermo Fisher Scientific. With industry skill and expertise built over nearly 40 years, we enhance the clinical research journey for pharmaceutical organizations of all sizes and budgets. Over the past five years, we’ve had the privilege of overseeing more than 2,800 studies, contributing to the advancement of medical science and patient care.
While we are proud of our involvement in over 575 drug approvals globally since 2017 and collaborations with the top 50 pharmaceutical companies, our driving force is to be a reliable partner in fast-tracking clinical research timelines. Our tested techniques and expertise drive advancements, so that our customers get back valuable time to drive breakthroughs.
The accelerator: Solutions built to surpass milestones
We understand the urgent need to accelerate clinical research. That’s why we employ a data-driven approach aimed at optimizing patient involvement. By proactively adjusting protocols and leveraging predictive analytics, we offer near real-time visibility across all study facets. This ensures timely issue resolution and a seamless journey from concept to approval for our customers.
Our comprehensive suite of services covers every aspect of drug development, empowering our clients to navigate the complexities of clinical research with confidence.
The finish line: Powering your success
It’s time for pharmaceutical developers to make clinical research timelines work for them, not against them. On this journey, a partnership with a trusted provider of CRO solutions like us becomes invaluable.
Together, we speed access of life-saving treatments to those in need — advancing health care to give back valuable time to patients.
It’s time for a partner that moves you forward.
Recommended for you