
Advancing New Treatments for Alopecia Areata
Our dermatology experts have conducted pivotal clinical studies for molecules on the market that can accelerate your alopecia areata treatments.
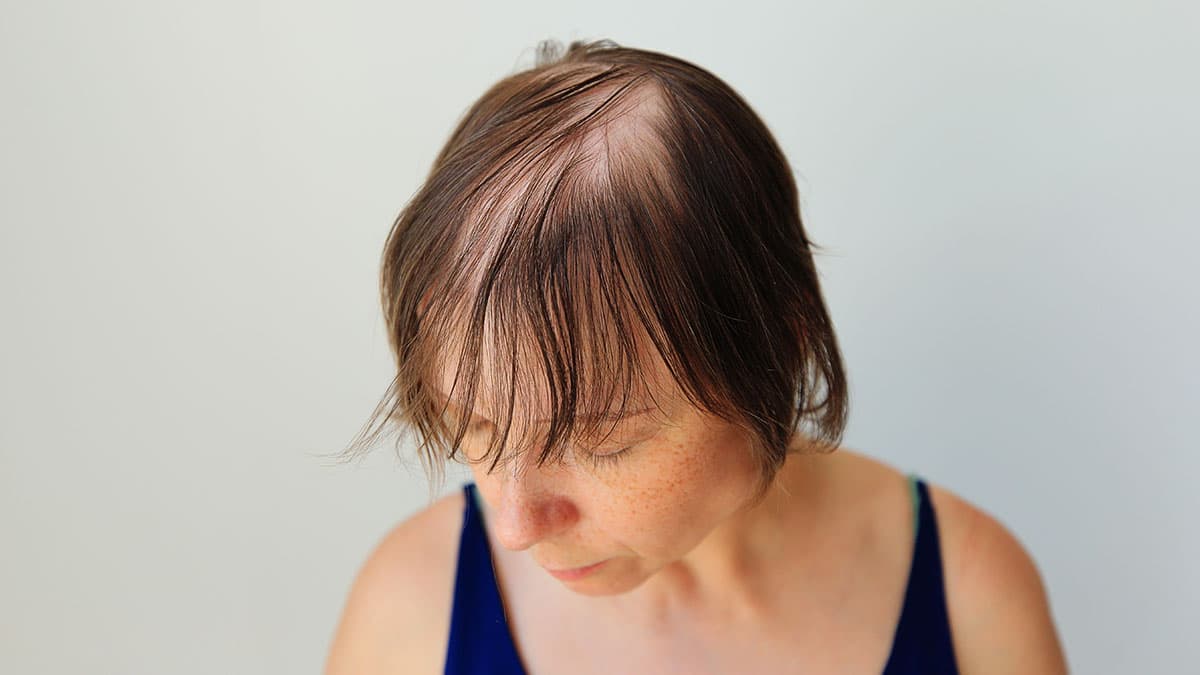
Alopecia areata (AA), also known as spot baldness, is a condition that causes hair loss from some or all areas of the body. The resulting bald spots may be patchy and vary in size, though hair loss may affect the entire scalp or all body hair. While there is no cure for the condition, in most cases the resulting hair loss is not permanent.
AA is considered a dermatology autoimmune disease, wherein the body fails to recognize its own cells and leads to subsequent immune-mediated destruction of the hair follicle. This distinguishes AA from the more common androgenetic alopecia (pattern baldness), which is related to genetic and hormonal factors.
Studies of the underlying pathology of AA have shown it is the result of a complex inflammatory pathway. In recent years, AA has followed in the footsteps of other dermatology indications — including psoriasis and atopic dermatitis — with growing research and clinical studies focusing on small molecules with broad cellular effects (JAK and PDE4 inhibitors) and cytokine-specific molecules antagonists (IL-23, Th2 and IL17) to treat the condition.
Our expertise in alopecia areata studies
As of July 2023, there were more than 60 planned or ongoing clinical trials for alopecia areata, involving nearly 20 pharmaceutical or biotech organizations. In general, ongoing research in AA is focused on molecules whose mechanism of actions and effectiveness have been proven in psoriasis and atopic dermatitis. The PPD™ clinical research business of Thermo Fisher Scientific has made significant contributions to these indications — our experts have conducted pivotal clinical studies for at least four molecules now on the market.
As the search for treatments for alopecia areata continues, it’s important for drug developers to understand the key pillars of several dermatology indications: the consistency of endpoints and the intrasite and intersite variability for raters’ assessed endpoints. We have developed unique artificial intelligence (AI) platforms to closely monitor clinical data from sites on a real-time basis and deliver the most relevant and accurate data for all the identified dermatology endpoints.
This is the result of the experience that our experts have developed through the successful delivery of dermatology clinical trials for 28 pharmaceutical and biotech companies across all regions, including North America, South America, Europe and Asia-Pacific, over the past five years. Our experience covers AA, but extends to a broad range of dermatology indications, including:
- Actinic keratosis
- Atopic dermatitis
- Bullous pemphigoid
- Dermatomyositis
- Epidermolysis bullosa
- Glabellar lines
- Hidradenitis suppurativa
- Pemphigus vulgaris
- Prurigo nodularis
- Pruritus, psoriasis
- Urticaria
Building alopecia areata registries
We’re able to bring sponsors deeper insights into AA through our association with CorEvitas, which is currently running an AA patient registry with advisement provided by industry-leading key opinion leaders. This registry is being developed with the intent of delivering data associated with long-term post-approval safety studies in the indication. CorEvitas will be deploying AA focus groups among physicians and patients to provide insights for pragmatic clinical trial designs.
Strong track record in dermatology research
With a deep understanding of the complexities of dermatology drug development, our clinical research services enable sponsors to accelerate timelines, reduce costs and perform the highest quality clinical trials targeting alopecia areata.
Our extensive experience in dermatological research encompasses more than 65 clinical trials worldwide involving more than 8,900 patients and 1,400 investigators. In recent years, we have developed a full set of technologies and systems that enable sponsors to conduct dermatology clinical trials without compromising scientific integrity or patient safety — whether those trials are conducted using traditional, hybrid or fully decentralized models.
Sponsors regularly tap into our clinical trial expertise, global network of sites and a large database of potential trial participants to advance dermatology trials. Whether your therapy for alopecia areata or other dermatology conditions is in the form of an oral, subcutaneous or topical treatment, we are committed to delivering the best approach to propel you to success.
Accelerate your alopecia areata treatments.
Recommended for you



