
Relieve Caregiver Burden, Improve Neurodegenerative Clinical Trials
Successful dementia trials must not only support the person with dementia but also the person taking care — yet most care partners are overburdened and unaware of trials. Here is how to solve for that.

For neurodegenerative disease research, the care partner, or caregiver, is equally important as the person with dementia. The care partners are the decision-makers for their loved ones with neurodegenerative disorders, handling everything from daily routines and medication management to doctor visits.
Yet, four out of five care partners of people with neurodegenerative disorders have never been informed of clinical trial opportunities, according to a survey of more than 250 caregivers conducted by the PPD™ clinical research business of Thermo Fisher Scientific.
Even with stronger awareness of studies, drug developers must contend with the inherent lack of trust between the dementia community and clinical trials.
There’s little time to lose. The global dementia population is set to triple by 2050, and our biotech and biopharma customers seek to move quickly to elevate their research and adopt proven strategies.
To overcome the inherent challenges in neurodegenerative clinical trials, sponsors must demonstrate a strong collaboration with caregivers.
They should look to clinical research partners already leading initiatives to raise awareness of studies, reduce care partner burden and enhance access to this important population.
Biotech and biopharma organizations that can do so stand poised to unlock a higher level of neurodegenerative clinical trials.
Our clinical research team has led more than 50 studies across a range of neurodegenerative indications, and is at the forefront of transformative strategies that close the gap between the clinical trial and the community ecosystems.
Based on our expertise and experience, we’ve developed a dynamic approach for sponsors to fortify their research into neurodegenerative diseases.
Recognize and relieve the caregiver burden.
In general, the majority of people with dementia will live at home under the care of a family member. The care partner takes on the responsibility of learning about the disease, understanding the different phases their loved one experiences, and attending every doctor visit.
Ideally, they also connect with community members in similar situations for support because the burden of caregiving takes its toll. According to research, dementia family care partners have elevated depressive and anxiety symptoms and are at greater risk for health issues such as cardiovascular diseases.
Our experts speak to investigators about the stressors of caring for a loved one with dementia and look for approaches to improve their quality of life and wellbeing. We know care partners benefit from access to a wellness professional, whether a psychologist, life coach, occupational therapist or social worker.
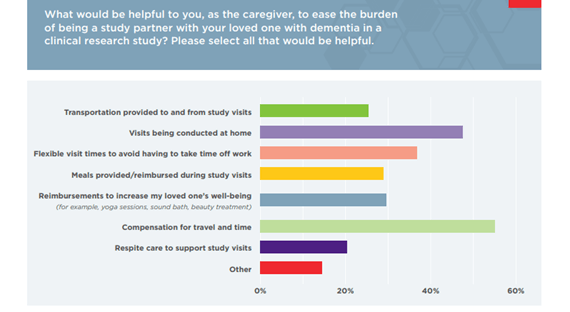
This connection to a wellness professional is a meaningful way for sponsors to reimburse care partners in countries that do not permit stipends, or to show support to care partners who provide transportation to appointments — particularly as a quarter of care partners say transportation is an impediment to research participation.
Build relationships with patient advocacy groups and community groups to improve awareness of studies.
Patient advocacy groups want face-to-face relationships with researchers. We have local teams across Europe and the U.S. communicating with community support groups, spending time with them and building rapport. It’s critical to build trust with these professionals because within the dementia community, care partners are reluctant to listen to clinical researchers or participate in a trial if the research partner is a stranger.
Case in point: When I started looking into patient advocacy groups in Portugal, where I reside, it took me a year to schedule a time to meet with a key group, in part due to the lack of trust between the community ecosystem and the clinical trial ecosystem. As I began talking with the patient advocacy group, I immediately encountered the negative thinking about trials, with sentiments like, “I don’t want to be a guinea pig.”
This particular advocacy group in Portugal has created a safe space for care partners to bond, drink coffee, get information and ask questions. As I’ve demonstrated my trust, genuine empathy and advocacy for the community and their loved ones, I now have an opportunity to communicate with them regularly.
I attended local memory cafés and with my team presented at a Patient Advocacy Group conference to educate professionals and care partners about clinical trials, the differences between the therapeutics and the risks and benefits. This year we will showcase our collaboration by hosting an event to address the prevention of dementia.
Keeping people at the heart of our efforts, our experts work closely with sponsors to collaborate with the care partner community and communicate trials in a meaningful way — enabling them to seek information, improve literacy about memory diseases and their different stages, and build confidence in studies.
Breakthroughs with care partners are imperative to advance knowledge, recruitment and retention in trials for Alzheimer’s disease and Parkinson’s disease, but they don’t happen overnight or with only the endgame in mind.
Through our efforts, we’ve not only improved awareness of neurodegenerative disease research, but also propelled our clients’ ability to reach, recruit and retain the core population.
Reexamine trial protocols to unburden people with dementia.
As the clinical research community becomes more visible, we must show that we’re not only listening to dementia care partners, but actively responding.
By tapping into their contract research organization’s (CRO) strong relationships with care partners and patient advisory groups, sponsors can develop more patient-centric trial protocols.
Practices we’ve seen deliver results include:
- Caregiver advisory boards. A best practice for patient-centered solutions is a patient advisory board. Neurodegenerative clinical research should incorporate both a patient advisory board, which works well for early stages of dementia, as well as a caregiver advisory board.
Alongside your CRO, a caregiver advisory board will offer guidance for sponsors to increase protocol viability and bring out-of-the-box thinking to achieve research goals. For example, our caregiver survey suggests that compensation in the form of meals provided during a study visit can positively influence study participation.
- Reduced protocol complexity. Participants on caregiver advisory boards can review study design and offer feedback on feasibility. Some of today’s dementia protocols are complex, with numerous assessments, blood tests, screening questions and more.
While well-intentioned, these appointments often ask for an unrealistic commitment from the person with dementia and their care partner. Approximately 50-60% of care partners note the overall time commitment affects their decision to participate in research. Care partner feedback enables sponsors to prioritize endpoints.
- Concierge and home health services. Concierge services are transformative for people with dementia because they bring the site to the patient and care partner. Via home health, investigators conduct assessments in the home of the person with dementia, saving time and money.
Nearly 50 percent of care partners we surveyed said visits conducted at the home would ease the burden of being a study partner. Another extension of this is to have trial sites located in a hospital, where the person with dementia already routinely visits.
Stay on the cutting edge of tactics that are transforming dementia research.
With talk of the coming “Alzheimer’s tsunami,” neurodegenerative clinical research must accelerate through transformative programs. Our experts have long been trailblazers in this field, from our clinical research unit in Orlando to new programs that partner with pharmacies to recruit candidates more effectively.
Some of the ways in which we’re leading the industry are:
- Dedicated clinical research unit in Orlando, Florida. Our Orlando clinic conducts research on a range of neurodegenerative disorders, from Alzheimer’s disease to frontal temporal lobe dementia, and has access to a large, aging patient population. As such, the clinic overcomes challenges that other dementia sites commonly face, such as repeated utilization – and exhausted patient pools.
Customers who tap into our Orlando clinic benefit from our innovative strategies and test-and-learn approach, whether we’re piloting a dedicated team member who conducts face-to-face outreach in the community or the clinic’s efforts to host events within the community. We scale successful tactics from Orlando across the U.S. and to other countries. This clinical research unit is also able to seamlessly transition persons with dementia into Phase II, peri- and post-approval and real-world studies, providing a true end-to-end solution.
- Targeted recruitment via retail health care. One example of our work to improve awareness of studies is leveraging retail health care for clinical trial recruitment. Imagine being able to recruit the ideal person with dementia by handing them a flyer about your study when they pick up their dementia medication at the corner Walgreens.
By recognizing which persons with dementia at a given pharmacy are taking the standard dementia medication, we can do a targeted recruitment campaign. And, our deep understanding of the care partner’s burden and motivations enables our outreach to be meaningful, not throwaway.
- Non-drug therapies. More technologies exist today for people with dementia, and in some clinical trials we’re experimenting with interventions like music therapy to boost mood, memory and communication. Think: an app like Vera that delivers personalized music therapy based on the demographics and interests of the person with dementia. A strong and natural stimulant, music activates long-term memory as well as all other areas of the brain – and inspires positive moments for both the person with dementia and their care partner.
We also follow global site networks like the World-Wide FINGERS Network, which focuses on non-drug therapies, to fold learnings into our clients’ protocols. The future of neurodegenerative clinical research will likely include a blend of pharmacological therapy and lifestyle interventions, and we’d be remiss to focus only on the former.
- Relationships with community organizations and physicians. Research shows that Hispanic and Black populations will experience disproportional increases in Alzheimer’s disease and related dementias. In addition to our work on the ground with patient advocacy groups globally, our experts examine how to identify and train physicians who treat the most vulnerable people with dementia. As we understand which doctors and neurologists people with dementia visit, we can create networks to communicate study information and ensure patient populations in trials mimic the real world.
Let’s close the gap between the community ecosystem and clinical trial ecosystem together.
The clinical research community used to focus on just the science, but it’s morphing. Our approach starts with the person rather than the condition, and as such we’ve built a dependable method for our customers to take neurodegenerative clinical research to the next level.
Caring for the care partner isn’t just the starting point — it will be the tipping point for research in this field.