
How COVID-19 is Changing the Approach for the Vaccine Development Process

Dr. Martina Kovac, medical lead for vaccines in medical and scientific strategy, presented at the Disease Prevention and Control Summit about innovative solutions that enable the acceleration of vaccine development in light of the current COVID-19 pandemic. This blog post highlights her key points.
COVID-19 has become one of the biggest challenges the world is facing today. The pandemic has placed a spotlight on how interconnected the world has become and will impact the vaccine market landscape moving forward. We have witnessed strong examples of collaboration, innovative technologies and partnerships that will enable new ways of conducting clinical development that can be used as a model in the future.
Using experience and collaboration in the vaccine development process
Developers are leveraging lessons learned from previous pandemics such as H1N1, Ebola and SARS. There are also unique private and public sector partnerships enabling scientific dialogue, accelerating clinical development and providing agility to scale up vaccine production.
For the first time, large pharmaceutical companies are coming together in partnership to expedite the development of vaccines and therapies. This momentum of global collaboration and connectivity has created communities to share know-how, expertise, tools and databases enhanced by artificial intelligence and digital platforms.
Rapid issuance of clinical trial guidance and expedited regulatory review and approval processes have been integral in the global mobilization of vaccine development. The hope is that this will continue with updated or newly created government policies to support public health and international cooperation. It’s encouraging that successful vaccines against COVID-19 could become available under an emergency use authorization in 2021.
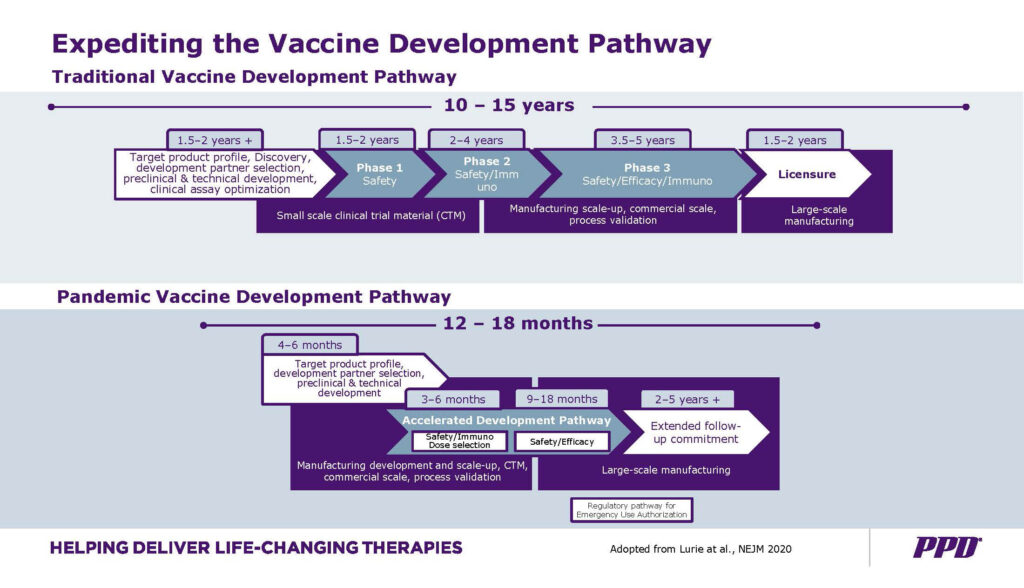
Accelerated vaccine development timelines
In a non-pandemic setting, the traditional approach for vaccine development is a slow process and can take 10 to 15 years to be approved. This is mainly due to the need to establish safety and efficacy through large-scale, long-term randomized controlled clinical trials, as well as thorough regulatory processes that can differ across geographic regions. There is also a high level of complexity behind the process validation of the vaccine commercial scale manufacturing process.
Developing a vaccine during a pandemic situation, however, requires faster initiation and execution of many steps in parallel, rather than sequentially as in a traditional approach. For example, the preclinical phase and selection of candidates could be shortened to four to six months, followed by accelerated clinical development with overlapping phases and adaptive study designs, such as combining Phases I and II or Phases II and III while maintaining safety first and high-quality standards for data integrity.
Established vaccine development expertise
Scientists are racing to develop COVID-19 vaccines on an unprecedented timeline and many developers are moving at a pace that has never been seen before. The current pandemic experience is transforming and shaping the way we conduct clinical trials.
With over 25 years of vaccine development experience, the PPD™ clinical research business of Thermo Fisher Scientific has rapidly adjusted to this evolving public health emergency. Our innovation and virtualization journey began well before the pandemic, enabling us to embrace and execute a wide variety of solutions.
For COVID-19 vaccine and therapeutic clinical studies, we have achieved start up in very short timelines by using innovative approaches. We combine our extensive therapeutic and laboratory expertise with our vast global resources to offer a distinctive caliber of vaccine development services and accelerate development with proven capabilities and dedicated resources.