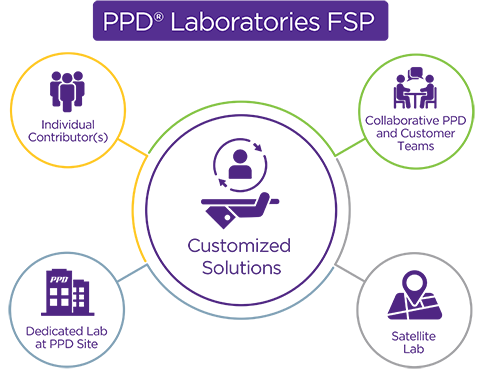
PPD® Laboratory Services, GMP Lab
lab services backed by three decades of CMC pharmaceutical testing experience
Our GMP lab provides high-quality analytical services for small molecules and biologics including active pharmaceutical ingredient, drug product, inhaled and other medical devices for all phases of drug development. As a leading provider of CMC lab services, we focus on responding to our clients’ needs with experienced staff, the latest technology, quality service and a high degree of flexibility.
We carry out CMC testing following current good manufacturing practices (cGMP) for all types of pharmaceutical products across all phases of development. Our highly qualified GMP lab scientists work with small molecules and biologics, including active pharmaceutical ingredients (API), drug products, inhaled products, cell and gene therapies, and drug-device combination products.
Our GMP lab can help shorten your development timelines
State of the art facilities and experienced personnel supporting
- Analytical development and validation for drug products and devices, starting materials, APIs, etc.
- Stability testing and storage
- Release and quality control testing
- Inhaled pharmaceutical and biologics testing
- Physiochemical characterization
- Specialized expertise in mass spectroscopy
- Potency bioassay and molecular biology applications
- Microbiology
- CMC advisory services
- Impurity identification and characterization
- Qualified person (QP) release services
- Infectivity